Katherine Unger Baillie has found that protein drugs, which derive from biological sources, represent some of the most important and effective biopharmaceuticals on the market. Some, like insulin, have been used for decades, while many more based on cloned genes are coming to market and are valued for their precise and powerful functions. Plant-made Antimicrobial Peptide Targets Dental Plaque and Gum Tissues.
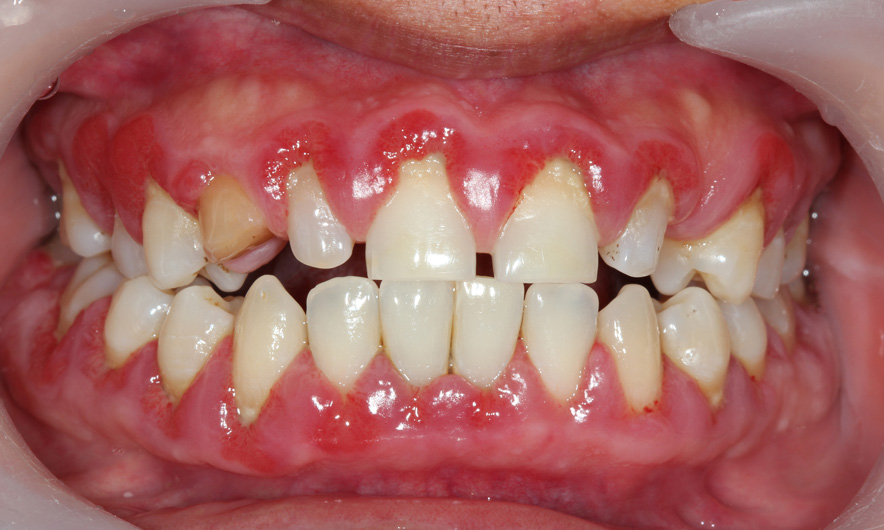
Yet the field of dental medicine has very few such drugs due to their high costs, and the ones that are used are delivered invasively, often through surgical procedures, to gum tissues.
Now, a report by University of Pennsylvania School of Dental Medicine scientists in the journal Biomaterials suggests a new approach for delivering a protein drug to treat and prevent oral diseases, including dental caries, commonly known as cavities. Using plants to produce antimicrobial peptides, the researchers were able to rapidly kill tooth-decay-causing bacteria and thwart their ability to form biofilms on a tooth-like surface with a single topical treatment. The peptides were even more effective when combined with an enzyme that degrades the matrix, which surrounds and protects bacteria residing inside biofilms.
In addition, the researchers demonstrated that these peptides, produced in a cost-effective manner in plants, could be taken up by periodontal and gingival cells, indicating that this novel delivery method could be useful in treating diseases that affect the gum tissues, perhaps by promoting wound healing or bone regeneration.
The platform is low-cost compared to the current means of producing biopharmaceuticals and presents a unique opportunity to develop an affordable therapeutic approach that simultaneously attacks disease-causing plaque and promotes gum health, the researchers said.
“As scientists we have many opportunities to develop breakthrough treatments but cost is a huge obstacle,” said Hyun (Michel) Koo, co-corresponding author on the study and professor in the Department of Orthodontics and divisions of Pediatric Dentistry and Community Oral Health in Penn Dental Medicine. “What makes this approach so exciting is not only the science but, because the production costs are low, the feasibility of getting the therapy to the population who truly needs yet can’t afford it.”
The work arose from a partnership between Koo and co-corresponding author Henry Daniell, director of translational research and professor in Penn Dental Medicine’s Department of Biochemistry. Koo was aware of Daniell’s groundbreaking plant-produced therapeutics for a number of important human infectious and inherited diseases. And Daniell learned that Koo had done extensive work on caries-causing biofilms, including searching for alternative approaches to degrade them or prevent them altogether.
“It was a synergism,” Daniell said. “Bringing our research together led to this new concept of a topical protein drug made in plants that can both kill bacteria and break down the oral biofilm.”
Dental caries predominantly affect children and adults of lower socioeconomic status and are responsible for more than $40 billion in health-care spending annually.
In the past, researchers have identified antimicrobial peptides that are potent killers of caries-causing bacteria. But these agents are expensive to make and have had limited success at killing bacteria protected by the extracellular matrix, as is found in dental plaque.
Meanwhile, other groups have investigated enzymes that can break down the biofilm matrix, and these, too, have had limited success at preventing dental caries by themselves.
In the new study, Koo, Daniell and colleagues tried a new approach, combining the antimicrobial peptides with the matrix-degrading enzyme.
To address the prohibitive cost of antimicrobial peptide production, the researchers turned to Daniell’s plant-based protein drug production platform. The process entails bombarding a plant leaf with gold particles coated in a cloned gene in order to reprogram the chloroplasts to synthesize the associated protein. In this case, the researchers coaxed plants to produce two different antimicrobial peptides, retrocyclin and protegrin. Both peptides have complex secondary structures, making them expensive to produce in the lab by traditional means. But the researchers found they could literally grow them in Daniell’s greenhouse and faithfully replicate their unique secondary structures in the plant’s leaves.
They then tested whether the plant-made agents could prevent creation of a biofilm. They exposed a saliva-coated tooth-like surface to the plant-made protegrin for 30 minutes, then exposed the surface to S. mutans cells along with sugar and found that it significantly impaired the ability of the bacterium to form a biofilm compared to an untreated surface.
To see whether the antimicrobials could act not just preventively but therapeutically, the researchers next exposed a pre-formed biofilm on the tooth-mimicking surface to either protegrin alone or a combination of protegrin and a matrix-degrading enzyme. The enzyme alone had no effect on the biofilm, and while the antimicrobial alone was able to kill some bacteria, the combination was powerful, able to degrade 60 percent of the matrix and killing even more bacteria.
“A single topical treatment was capable of disrupting the biofilm,” Koo said. “It’s effectiveness was comparable to chlorhexidine, which is considered the ‘gold standard’ for oral antimicrobial therapy.”
Beyond topical-drug delivery, Daniell’s lab has been investigating molecular “tags” to route protein drugs to human cells to treat several diseases. In this context, delivering growth hormones or other such drugs to gum tissues for wound healing or bone regeneration is of paramount importance to enhance oral health. Their study found that the plant-made antimicrobial peptides could be taken up by human cells in the oral cavity.
“This was unexpected,” Daniell said. “The antimicrobials didn’t harm any of the human cells in gum tissues but had an unusual ability to go across the cell membranes of periodontal and gingival cells. This opens up a completely new field for drug delivery with a topical agent.”
A collaboration with Johnson & Johnson Consumer Inc. will enable Koo and Daniell to continue optimizing their antimicrobial-enzyme production system. One possibility, they note, is to create a chewing gum laced with antimicrobial peptides that could be slowly released as one chews. Alternatively, for Asian cultures where betel leaf chewing is common, the researchers may investigate the possibility of growing these peptides in that plant to promote oral health.
Additional coauthors on the study included co-first authors Yuan Liu and Aditya C. Kamesh and Yuhong Xiao, Victor Sun and Michael Hayes, all of Penn Dental Medicine.
The work was supported by the National Institutes of Health and the Bill & Melinda Gates Foundation.